Medicine and Medical Device
DRUG, MEDICAL CONSUMPTION, MEDICAL DEVICE IMPORT & EXPORT
Area Of Duty & Responsibility
Our company is authorized to purchase and sell medicines, medical supplies and medical devices in Türkiye and abroad, as well as to supply medicinal products for human use that are not available in the market in our country for various reasons.
In addition, it is among the duties of our company to take the role of a supplier in public procurements between countries and to supply the materials needed for hospitals operating abroad under the Ministry of Health.
Laws and Regulations
- USHAŞ, which was established in accordance with the second paragraph of Annex 2 of the Decree Law No. 663, entered into force by being published in the Official Gazette dated 03.08.2018 and numbered 30498, is authorized to supply medicines, devices and medical supplies.
- As of 10.01.2020, it has been authorized to obtain human medicinal products from abroad that are not licensed and / or licensed in our country for various reasons.
- USHAS, except for the provisions of the criminal ban and tender 4/1/2002 dated: 4734 public procurement law, 5/1/2002 dated; public enterprises and public procurement contracts law no.4735 dated the decree No. 233 dated 18.06.1984 are not subject to.
Ministry of Public Health General Directorate, Microbiology Reference Laboratories and Biological Products Department and Bioeksen R&D Technologies Ltd. in partnership, we developed the COVID- 19 diagnostic kit for molecular testing of SARS-COV2 based on a reference protocol published by the WHO on January 17, 2020. The title of the reference method is “Diagnostic detection of Wuhan coronavirus 2019 with real-time RT-PCR”.
Our Products
- COVID-19 Diagnostic Kits
PCR Diagnostic Kits
Rapid Antigen Diagnostic Kits - Medical Devices
Ventilator Device
High Flow Oxygen Therapy Device - Medicines
- Personal Protective Equipment
Masks, aprons, overalls, gloves, etc.
It was developed in scope and produced “rapid viral nucleic acid isolation and diagnostic kit” to be produced by the intellectual property rights and / or the commercialization rights of Türkiye Institutes of Health Administration (TÜSEB), the production of goods and / or commercialization rights exclusively indefinitely on 02/03/2020 handed over to USHAŞ.
Medicines
The Pharmaceuticals and Medical Devices Operations Department carries out the deliveries of all kinds of human medical products (medications) needed under GDP (good transportation conditions) to domestic and foreign buyers by importing them from abroad and supplying them domestically. The Turkish pharmaceutical industry is a strong structure that can produce the drugs needed in our country with local resources at a rate of 70% on a box basis. These drugs are highly effective, reliable and can be produced more economically than many competing countries. For this reason, it is our priority as USHAŞ to supply the pharmaceutical demands coming from abroad by prioritizing the products of the domestic manufacturers, to export abroad and to provide foreign currency inflow to our country, while supporting the domestic manufacturer.
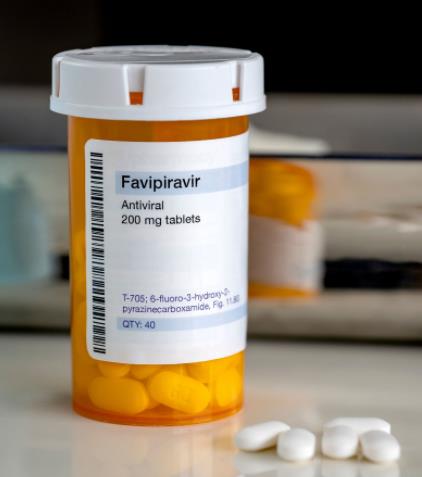
Necessary information
Drug Tracking System (ITS) Number
8680001564415
Product Tracking System (ÜTS) Number
2667269356361
USHAŞ ULUSLARARASI SAĞLIK HİZMETLERİ A.Ş. Üniversiteler Mah. Dumlupınar Blv. 6001. Cad. Sağlık Kampüsü Kat:8 06800 Çankaya ANKARA
Ankara Kurumlar V.D. 8940697604
Contacts:







